POS AM: Post-effective amendment to a registration statement that is not immediately effective upon filing
Published on July 29, 2025
As filed with the Securities and Exchange Commission on July 29, 2025
Registration No. 333-276590
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
POST-EFFECTIVE AMENDMENT NO. 1 TO FORM S-1 ON
Form S-3
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
ENVOY MEDICAL, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 86-1369123 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification Number) |
4875 White Bear Lake Parkway
White Bear Lake, MN 55110
Tel: (877) 900-3277
(Address, including zip code, and telephone number, including area code, of principal executive offices)
Brent T. Lucas
Chief Executive Officer
Envoy Medical, Inc.
4875 White Bear Lake Parkway
White Bear Lake, MN 55110
Tel: (877) 900-3277
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Please send copies of all communications to:
Andrew M. Nick
Edward M. Peilen
Fredrikson & Byron, P.A.
60 South Sixth Street, Suite 1500
Minneapolis, Minnesota 55402
Tel: (612) 492-7000
Approximate date of commencement of proposed sale to the public: From time to time after the effectiveness of the Registration Statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box: o
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box: þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. o
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ | Smaller reporting company þ | Emerging growth company þ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. o
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Explanatory Note
On January 18, 2024, the registrant initially filed a registration statement with the Securities and Exchange Commission (the “Commission”) on Form S-1 (Registration No. 333-276590), which was declared effective by the Commission on May 2, 2024 (the “Form S-1”), to register the issuance of up to 21,954,103 shares of Class A common stock, $0.0001 par value per shares (“Class A Common Stock”), the resale of up to 21,206,360 shares of Class A Common Stock by the Selling Securityholders named in the prospectus, and the resale of up to 3,874,394 warrants by the selling warrantholders named in the prospectus.
This Post-Effective Amendment No. 1 to Form S-1 on Form S-3 is being filed by the registrant to convert the Form S-1 into a registration statement on Form S-3, and contains an updated prospectus relating to the offering and sale of the shares that were registered for resale on the Form S-1.
All filing fees payable in connection with the registration of the shares of the common stock covered by the Form S-1 were previously paid by the registrant.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION DATED July 29, 2025 |

ENVOY MEDICAL, INC.
Up to 17,376,177 Shares of Class A Common Stock Issuable Upon Exercise of Warrants
Up to 3,588,406 Shares of Class A Common Stock Issuable Upon Conversion of Series A Preferred Stock
10,920,411 Shares of Class A Common Stock
3,209,511 Warrants
This prospectus relates to the issuance by us of up to an aggregate of 20,964,583 shares of our Class A common stock, $0.0001 par value per share (“Class A Common Stock”), consisting of:
| (i) | up to 14,166,666 shares of Class A Common Stock that are issuable upon the exercise of 14,166,666 warrants (“Public Warrants”) originally issued by our predecessor company, Anzu Special Acquisition Corp I (“Anzu”), as part of its initial public offering (“IPO”) of units at a price of $10.00 per unit, with each unit consisting of one share of Anzu Class A Common Stock and one-third of one Public Warrant; |
| (ii) | up to 3,209,511 shares of Class A Common Stock that are issuable upon the exercise of 3,209,511 warrants (“Shortfall Warrants” and, together with the Public Warrants, the “Warrants”) issued to the Meteora FPA Parties (as defined below) for no additional consideration pursuant to the Forward Purchase Agreement, dated April 17, 2023 (as amended to date, the “Forward Purchase Agreement”) by and among Anzu, Envoy Medical Corporation (“Legacy Envoy”) and Meteora Special Opportunity Fund I, LP (“MSOF”), Meteora Capital Partners, LP (“MCP”), Meteora Select Trading Opportunities Master, LP (“MSTO”) and Meteora Strategic Capital, LLC (“MSC”) (with MCP, MSOF, MSTO and MSC collectively as the “Meteora FPA Parties”); |
| (iii) | up to 1,849,276 shares of Class A Common Stock issuable upon conversion of 2,126,667 shares of our Series A Convertible Preferred Stock, par value $0.0001 per share (“Series A Preferred Stock”), held by Anzu SPAC GP I LLC (the “Sponsor”); |
| (iv) | up to 869,565 shares of Class A Common Stock issuable upon conversion of an aggregate of 1,000,000 shares of Series A Preferred Stock held by AICP III L.P., Anzu Industrial Capital Partners III, L.P. and Anzu Industrial Capital Partners III QP, L.P. (collectively, the “PIPE Investors”), each an affiliate of the Sponsor; and |
| (v) | up to 869,565 shares of Class A Common Stock issuable upon conversion of 1,000,000 shares of Series A Preferred Stock, which were issued to GAT Funding, LLC (“GAT”) in connection with the Closing in exchange for the Legacy Envoy Bridge Note (as defined below) at a price of $10.00 per share and have a conversion price of $11.50 per share. |
This prospectus also relates to the offer and sale from time to time by the Selling Securityholders named in this prospectus (each a “Selling Securityholder” and, collectively, the “Selling Securityholders”) of up to 3,209,511 Shortfall Warrants and up to 17,718,328 shares of Class A Common Stock, consisting of:
| (i) | up to 3,209,511 shares of Class A Common Stock that are issuable upon the exercise of 3,209,511 Shortfall Warrants issued to the Meteora FPA Parties for no additional consideration pursuant to the Forward Purchase Agreement; |
| (ii) | up to 1,849,276 shares of Class A Common Stock issuable upon conversion of 2,126,667 shares of Series A Preferred Stock, held by the Sponsor, which have a stated value of $10.00 per share and a conversion price of $11.50 per share; |
| (iii) | up to 869,565 shares of Class A Common Stock issuable upon conversion of an aggregate of 1,000,000 shares of Series A Preferred Stock held by the PIPE Investors, which have a stated value of $10.00 per share and a conversion price of $11.50 per share; |
| (iv) | up to 869,565 shares of Class A Common Stock issuable upon conversion of 1,000,000 shares of Series A Preferred Stock held by GAT, which have a stated value of $10.00 per share and a conversion price of $11.50 per share; |
| (v) | an aggregate of 125,000 shares of Class A Common Stock held by former directors of the Company; |
| (vi) | an aggregate of 10,795,411 shares of Class A Common Stock held by certain of Legacy Envoy’s former directors, officers and 5% or greater shareholders (collectively, the “Key Seller Stockholders”). |
We will not receive any proceeds from the sale of Shortfall Warrants or Class A Common Stock by the Selling Securityholders pursuant to this prospectus. Each Public Warrant entitles the holder thereof to purchase one share of Class A Common Stock at a price of $11.50 per share, and the Shortfall Warrant entitles the holder thereof to purchase one share of Class A Common Stock at a price of $1.50 per share, subject to adjustment. We will receive up to $167.7 million from the exercise of the Warrants, assuming the exercise in full of all of the Warrants for cash, but not from the sale of the shares of Class A Common Stock issuable upon such exercise. We expect to use any such proceeds for general corporate purposes, which may include acquisitions and other business opportunities. As of the date of this prospectus, the Public Warrants are “out of the money”, which means that the trading price of the shares of Class A Common Stock underlying the Public Warrants is below the $11.50 exercise price of the Public Warrants. For so long as the Public Warrants remain “out of the money,” we do not expect warrantholders to exercise their warrants and, therefore, we do not expect to receive cash proceeds from any such exercise. As a result, we may need to secure additional debt or equity financing. There can be no assurance that we will be successful in acquiring additional funding at levels sufficient to fund our operations or on terms favorable to us. If we are unable to raise sufficient financing when needed or events or circumstances occur such that we do not meet our strategic plans, we may be required to reduce certain discretionary spending, be unable to develop new or enhanced production methods, or be unable to fund capital expenditures, which could have a material adverse effect on our financial position, results of operations, cash flows, and ability to achieve our intended business objectives. To the extent that the market prices of the Class A Common Stock exceeds the exercise price of the Warrants, warrantholders may exercise their Public Warrants and Shortfall Warrants, respectively, and sell the underlying Class A Common Stock, which may have negative impact on the market prices of the Class A Common Stock.
We have registered the securities for resale pursuant to the Selling Securityholders’ registration rights under certain agreements between us and the Selling Securityholders. Our registration of the securities covered by this prospectus does not mean that the Selling Securityholders will offer or sell any of their Shortfall Warrants or Class A Common Stock. The Selling Securityholders may offer, sell or distribute all or a portion of their Shortfall Warrants and Class A Common Stock publicly or through private transactions at prevailing market prices or at negotiated prices. We will not receive any proceeds from the sale of Shortfall Warrants or Class A Common Stock by the Selling Securityholders pursuant to this prospectus. We provide more information about how the Selling Securityholders may sell their Shortfall Warrants and Class A Common Stock in the section entitled “Plan of Distribution.”
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”), and are subject to reduced public company reporting requirements. This prospectus complies with the requirements that apply to an issuer that is an emerging growth company.
Our Class A Common Stock and Public Warrants are listed on the Nasdaq Capital Market (“Nasdaq”) under the symbols “COCH” and “COCHW,” respectively. On , 2025, the closing price of our Class A Common Stock was $ and the closing price for our Public Warrants was $ .
Investing in our securities involves risks. You should carefully review the risks and uncertainties described under the heading “Risk Factors” beginning on Page 7 of this prospectus, any applicable prospectus supplement or any related free writing prospectus, and in any documents incorporated by reference herein or therein before investing in our securities.
We may amend or supplement this prospectus from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements carefully before you make your investment decision.
The Selling Securityholders may sell the securities directly to or through underwriters or dealers, and also to other purchasers or through agents on a continuous or delayed basis. The names of any underwriters or agents that are included in a sale of securities to you, and any applicable commissions or discounts, will be stated in any accompanying prospectus supplement. In addition, the underwriters, if any, may over-allot a portion of the securities.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION NOR HAS THE SECURITIES AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY ISACRIMINAL OFFENSE.
The date of this prospectus is , 2025.
TABLE OF CONTENTS
i
This prospectus is part of a registration statement on Form S-3 that Envoy Medical, Inc., a Delaware corporation, which is also referred to as the “Company,” “Envoy Medical,” “we,” “us,” “ourselves” and “our,” has filed with the United States Securities and Exchange Commission (the “SEC”) using a “shelf” registration procedure. Under this procedure, we may offer and sell at any time and from time to time, in one or more offerings, any combination of the securities described in this prospectus.
Any prospectus supplement may add, update, or change information contained in this prospectus. Any statement that we make in this prospectus will be modified or superseded by any inconsistent statement made by us in any prospectus supplement. The information in this prospectus is accurate as of its date. Additional information, including our financial statements and the notes thereto, is incorporated in this prospectus by reference to our reports filed with the SEC. Therefore, before you invest in our securities, you should carefully read this prospectus and any prospectus supplement relating to the securities offered to you together with the additional information incorporated by reference in this prospectus and any prospectus supplement (including the documents described under the heading “Where You Can Find More Information” and “Documents Incorporated by Reference” in both this prospectus and any prospectus supplement).
This prospectus incorporates by reference, and any prospectus supplement or free writing prospectus may contain and incorporate by reference, market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. In addition, the market and industry data and forecasts that may be included or incorporated by reference in this prospectus, any prospectus supplement or any applicable free writing prospectus may involve estimates, assumptions and other risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” contained in this prospectus, the applicable prospectus supplement and any applicable free writing prospectus, and under similar headings in other documents that are incorporated by reference into this prospectus. Accordingly, you should not place undue reliance on this information.
You should rely only on the information contained in or incorporated by reference in this prospectus or any prospectus supplement. Neither we nor the Selling Securityholders have authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. Neither we, the Selling Securityholders nor anyone acting on our behalf is making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information incorporated by reference or provided in this prospectus or any prospectus supplement is accurate as of any date other than the date on the front of those documents.
ii
This summary highlights information contained in other parts of this prospectus and in the documents we incorporate by reference. Because it is only a summary, it does not contain all of the information that you should consider before investing in our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere or incorporated by reference in this prospectus. You should read all such documents carefully, especially the risk factors and our consolidated financial statements and the related notes included or incorporated by reference in this prospectus, before deciding to buy shares of our common stock.
About the Company
We are a hearing health company focused on providing innovative medical technologies across the hearing loss spectrum. Our technologies are designed to shift the paradigm within the hearing industry and bring both providers and patients the hearing devices they desire. We are dedicated to pushing beyond the status quo to provide patients with improved access, usability, independence, and quality of life. We were founded in 1995 to create a fully implanted hearing device that leveraged the natural ear - not an artificial microphone - to pick up sound. The ear itself is an ideal way to capture sound from our environment.
To leverage the natural ear’s benefits, an implanted sensor was created to pick up incoming sound energy from the ossicular chain (i.e., the three tiny hearing bones that connect the eardrum to the cochlea). The sensor absorbs the mechanical energy from ossicular chain and turns it into a signal that can be processed, improved, and increased for a patient’s particular hearing needs.
Our first product, the Esteem Fully Implanted Active Middle Ear Implant (“Esteem FI-AMEI”), received FDA approval in 2010. The Esteem FI-AMEI remains the only FDA approved fully implanted active hearing device on the market. The Esteem FI-AMEI failed to gain commercial traction, primarily because the Centers for Medicaid and Medicare Services (“CMS”) classified it as a hearing aid and therefore not eligible for coverage. At an average total price (i.e., device and surgery) of over $25,000, very few individuals were willing or able to pay out-of-pocket for the Esteem FI-AMEI. We believe hearing aid classification is improper for the Esteem FI-AMEI and we continue to work towards having the Esteem FI-AMEI properly classified as a Fully Implanted Active Middle Ear Implant.
Despite the commercial challenges of the Esteem FI-AMEI, roughly 1,000 devices were implanted globally. Some devices were implanted in the early 2000s during clinical trials, providing us with nearly two decades of experience with its implantable sensor technology. Throughout our experience, our sensor technology proved a viable alternative to external or implanted microphones.
In late 2015, we made the decision to shift our focus from the Esteem FI-AMEI to a new product that would leverage our sensor technology and incorporate it into a cochlear implant. As a result, we have developed the investigational fully implanted Acclaim CI. We now believe we have the possibility to disrupt the cochlear implant market currently dominated by a small number of incumbents.
Our Product Candidate
Cochlear Implants - Fully Implanted vs. Partially Implanted
The cochlea converts vibrations from the ossicular chain into nerve signals that are transmitted through the auditory nerve for processing by the brain. Cochlear implants use electronic signals to stimulate the auditory nerve.
1
Partially implanted cochlear implants have two main components: a large external component that sits on or behind the patient’s ear and a surgically implanted internal component. The external component contains a microphone, sound processer, and batteries. A magnetic coil on the external component lines up with an internal magnetic coil in the internal component. The signal from the external component is transferred to the internal coil where it is delivered to the electrode array, which is implanted in the cochlea, to electrically stimulate the cochlea.
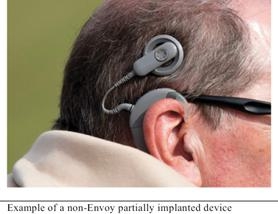 |
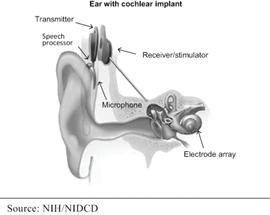 |
The Acclaim CI is fully implanted and does not have the need for any external component to be worn on the ear. Unlike partially implanted devices, the fully implanted Acclaim CI uses the ear to capture sound via a piezoelectric sensor that is implanted in the middle ear. The sound processor and power source are also implanted.
|
||
| CAUTION: Investigational Device – Limited by Federal (or United States) Law to Investigational Use. | ||
 |
Acclaim CI - A Breakthrough Device
The fully implanted Acclaim CI received the Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) in 2019. However, the process of medical device development is inherently uncertain and there is no guarantee that this designation will accelerate the timeline for approval or make it more likely that the Acclaim CI will be approved.
Hearing loss is currently an irreversible and debilitating human condition. Significant hearing loss is correlated with increased anxiety, depression, social isolation, falls, and other costly health issues. An article published in the journal Acta Otorhinolaryngol Italica in June 2016 suggests that untreated or undertreated hearing loss correlates with earlier loss of cognitive function and poorer cardiovascular health.1 While some solutions for hearing loss already exist (e.g., hearing aids, traditional cochlear implants) these have inherent limitations in being fully or partially external, which may limit patients in initial time to adoption, hours of use during the day (inherent compliance restrictions), lifestyle, or quality of life.
| 1 | Source: Fortunato S, et al.; A Review of New Insights on the Association Between Hearing Loss and Cognitive Decline in Ageing; Acta Otorhinolaryngologica Italica (Jun 2016), finding that increasing evidence has linked age related hearing loss to more rapid progression of cognitive decline and incidental dementia and that many aspects of daily living of elderly people have been associated to hearing abilities, showing that hearing loss affects the quality of life, social relationships, motor skills, psychological aspects and function and morphology in specific brain areas. |
2
We believe that the Acclaim CI will be able to offer hearing benefit over the patient’s baseline condition and may also offer other important advantages over alternative hearing loss treatments, such as:
| ● | Increased daily usage. We believe that the fully implanted nature of the Acclaim CI may facilitate an increase in daily usage over other types of cochlear implants because the device can be used 24-hours a day. | |
| ● | Hearing at night. Unlike other types of available cochlear implants, the Acclaim CI can be used at night. This capability may support audibility of alarms, sirens, telephones, and other people for an added sense of security while they sleep. | |
| ● | Hearing in and around water. Patients using the Acclaim CI will not need to worry about removing their device when showering, at the beach, or swimming laps. They will also not need to worry about damaging the device if caught in the rain. | |
| ● | Hearing in active situations. A patient using the Acclaim CI will not need to worry about the external processor falling off during exercise or other physical activities. The patient will not need to preemptively remove the device prior to engaging in these types of activities, thus retaining audibility of the surrounding environment. |
| ● | Lowered battery maintenance. Other cochlear implants require near-daily battery replacement or battery charging. In addition to the logistical hassle of worrying about keeping the batteries charged, this can be challenging for patients who have issues with dexterity or neuropathy, as the batteries and components are small and can be hard to handle. The Acclaim CI is designed with a battery contained within the implanted system components intended to be charged wirelessly through the skin. The Acclaim CI battery is expected to last for several days between charges and will not require the patient to use or handle small components like current cochlear implant systems do. | |
| ● | No need for backup or secondary processors. Many patients who have partially implanted cochlear implants with external hardware desire or need a backup processor. The backup processor provides the patient with a sense of security because they know if their primary processor is lost or damaged, they will be left without hearing for a period of time while they wait for a replacement. In addition, lost or damaged components can be expensive to replace, with the cost of replacement often not covered by insurance. The Acclaim CI processor is implanted and therefore not susceptible to damage, discomfort or issues associated with moisture, germs, dirt, or other external causes of loss or physical damage due to having an externally worn processor. | |
| ● | Use of equipment and accessories. The externally worn components of currently available cochlear implants can make wearing equipment or accessories difficult for existing cochlear implant patients. For example, wearing helmets, hats, headphones, stethoscopes, or other accessories can interfere with the placement of the external components and cause “coil offs” or prevent the patient from using the device altogether. | |
| ● | Earlier adoption of cochlear implant technology from reduced stigma. For many potential users of hearing instruments like hearing aids and cochlear implants, the perception of stigma associated with those technologies can prevent or delay the adoption of the technology. We believe that the Acclaim CI, with no externally worn components, may help reduce or perhaps even eliminate such stigma. We believe we can increase penetration rates for adult cochlear implants in the U.S. | |
| ● | Potential to significantly reduce overall costs while improving net healthcare outcomes. We believe a fully implanted cochlear implant could reduce cochlear implant costs over time by eliminating costly external components that are frequently replaced at the expense of the patient, the insurer, Medicare, or other third-party payor. There is also reason to believe that increasing compliance and use of cochlear implants, reducing time to adoption for candidates, and helping to support safety and security by providing the ability for true all-day hearing may improve the net healthcare outcome for society over time. |
3
The Acclaim CI is implanted by a surgeon through a procedure that we believe will average around two and a half to three hours under general anesthesia. We expect that patients may experience mild to moderate discomfort after the procedure. A four to eight week waiting period is required before the Acclaim CI can be activated to allow the middle ear to heal and fluid from surgery to dissipate. It is expected that the Acclaim CI battery pack will be replaced every 8-12 years via a less invasive surgical procedure that only replaces the Acclaim CI battery pack in the pectoral region (i.e., the whole system does not need to be replaced, just the Acclaim CI battery pack).
All of the competitive advantages referred to above require that the Acclaim CI obtain FDA approval in its current form and substantially on our planned timeline. If FDA approval is materially delayed for any reason, it is possible that competitors will offer products with similar features before we are able to market the Acclaim CI.
Business Combination
In September 2023, we completed the Business Combination pursuant to the Business Combination Agreement between Anzu and Legacy Envoy. As contemplated by the Business Combination Agreement: (a) each share of Legacy Envoy Preferred Stock issued and outstanding immediately prior to the Closing was converted into shares of Legacy Envoy Common Stock; (b) each share of Merger Sub Common Stock issued and outstanding immediately prior to the Closing was converted into and exchanged for one share of Legacy Envoy Common Stock; (c) each outstanding option to purchase shares of Legacy Envoy Common Stock outstanding as of immediately prior to the Closing was cancelled in exchange for nominal consideration; (d) each outstanding warrant to purchase shares of Legacy Envoy Common Stock outstanding as of immediately prior to the Closing automatically, depending on the applicable exercise price, was cancelled or exercised on a net exercise basis and converted into shares of Legacy Envoy Common Stock in accordance with its terms; (e) each outstanding Legacy Envoy convertible promissory note was automatically converted into shares of Legacy Envoy Common Stock in accordance with its terms; (f) each share of Legacy Envoy Common Stock issued and outstanding immediately prior to the Closing was cancelled and converted into the right to receive a number of shares of our Class A Common Stock equal to the Exchange Ratio; (g) the Sponsor forfeited 5,510,000 shares of Anzu Class B Common Stock and all 12,500,000 private warrants pursuant to the Sponsor Support Agreement; (h) the Sponsor exchanged 2,500,000 shares of Anzu Class B Common Stock for 2,500,000 shares of our Series A Preferred Stock; (i) an aggregate of 2,615,000 shares of Anzu Class B Common Stock held by the Sponsor and Anzu’s former independent directors automatically converted into our Class A Common Stock; (j) the Sponsor transferred an aggregate of 490,000 shares of our Class A Common Stock to the Legacy Forward Purchasers and the Extension Support Parties pursuant to the Side Letter Agreements and Extension Support Agreements, respectively; and (k) the Company issued an aggregate of 8,512 shares of Class A Common Stock to the Meteora FPA Parties pursuant to the Forward Purchase Agreement.
Risk Factors
Our operations and financial results are subject to various risk and uncertainties. Before deciding to invest in our securities, you should carefully consider the factors described under “Risk Factors” beginning on Page 7 of this prospectus, as well as the other information included elsewhere in this prospectus, and the risk factors described under “Part I, Item 1A. Risk Factors” in our most recent Annual Report on Form 10-K and in any subsequently-filed Quarterly Reports on Form 10-Q, and those contained in our other filings with the SEC that are incorporated by reference in this prospectus. Any of the foregoing risk factors could adversely affect our business, results of operations, financial condition and prospects. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also adversely affect our business operations.
Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenues during our last fiscal year, we qualify as an emerging growth company as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in 2012. As an emerging growth company, we expect to take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| ● | being permitted to present only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion andAnalysis of Financial Condition and Results of Operations” disclosure in this prospectus; |
4
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-OxleyAct of 2002, as amended; |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; |
| ● | exemption from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; and |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We elected to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBSAct.
Implications of Being a Smaller Reporting Company
Rule 12b-2 of the Exchange Act defines a “smaller reporting company” as an issuer that is not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent that is not a smaller reporting company and that:
| ● | had a public float of less than $250 million as of the last business day of its most recently completed second fiscal quarter, computed by multiplying the aggregate worldwide number of shares of its voting and non-voting common equity held by non-affiliates by the price at which the common equity was last sold, or the average of the bid and asked prices of common equity, in the principal market for the common equity; or |
| ● | in the case of an initial registration statement under the Securities Act, or the Exchange Act of 1934, as amended, which we refer to as the Exchange Act, for shares of its common equity, had a public float of less than $250 million as of a date within 30 days of the date of the filing of the registration statement, computed by multiplying the aggregate worldwide number of such shares held by non-affiliates before the registration plus, in the case of a Securities Act registration statement, the number of such shares included in the registration statement by the estimated initial public offering price of the shares; or |
| ● | in the case of an issuer whose public float as calculated under the previous two bullet points was zero or less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year for which audited financial statements are available. |
We believe that we are a smaller reporting company, and as such that we will not be required and may not include a Compensation Discussion and Analysis section in our proxy statements; we will provide only two years of financial statements; and we need not provide the table of selected financial data. We also will have other “scaled” disclosure requirements that are less comprehensive than issuers that are not smaller reporting companies. These “scaled” disclosure requirements may make our securities less attractive to potential investors, which could make it more difficult for our security holders to sell their securities.
Corporate Information
Our principal executive office is located at 4875 White Bear Parkway, White Bear Lake, Minnesota 55110. Our telephone number is (877) 900-3277, and our website is www.envoymedical.com. The information contained on or accessible through our website is not incorporated by reference into, and should not be considered part of, this prospectus or the information incorporated herein by reference.
Where You Can Find More Information
You should rely only on the information contained in this prospectus or incorporated herein by reference. We have not authorized anyone to provide you with additional information or information different from that contained in this prospectus filed with the SEC or incorporated herein by reference. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, the common shares and pre-funded warrants only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this document, regardless of the time of delivery of this prospectus or any sale of the common shares and pre-funded warrants. Our business, financial condition, results of operations, and prospects may have changed since the date hereof.
5
THE OFFERING
| Issuer | Envoy Medical, Inc. | |
| Common Shares Offered by the Selling Securityholders | Up to 17,718,328 shares of Class A Common Stock | |
| Common Shares Outstanding | 21,520,392 sharse of Class A Common Stock | |
| Use of Proceeds | We will use any proceeds from the exercise of the Warrants for general corporate purposes. We will not receive any proceeds from the sale of the common shares being offered for sale by the Selling Securityholders. | |
| Plan of Distribution | The Selling Securityholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. Registration of the common stock covered by this prospectus does not mean, however, that such shares necessarily will be offered or sold. See the section entitled “Plan of Distribution.” | |
| Dividend Policy | We have paid no dividends on the common shares to date, and we do not expect to pay dividends on our common shares in the foreseeable future. | |
| Listing and Trading Symbol | Our Class A Common Stock and Public Warrants are listed on the Nasdaq Capital Market (“Nasdaq”) under the symbols “COCH” and “COCHW,” respectively. | |
| TransferAgent and Registrar | Equiniti Trust Company, LLC. | |
| Risk Factors | You should carefully read and consider the information set forth under the heading “Risk Factors” and all other information set forth in this prospectus before deciding to invest in our common shares, pre-funded warrants, and warrants. |
The number of shares of our common stock to be outstanding is based on 21,520,392 shares of Class A Common Stock outstanding as of July 28, 2025 and excludes as of such date:
| ● | 14,166,666 shares of Class A Common Stock issuable upon exercise of the Public Warrants with an exercise price of $11.50 per share; |
| ● | 3,209,511 shares of Class A Common Stock issuable upon exercise of the Meteora Warrants with an exercise price of $1.50 per share, subject to adjustment; |
| ● | 3,500,000 shares of Class A Common Stock issuable upon exercise of the warrants held by GAT with a weighted average exercise price of $1.90 per share; |
| ● | 3,209,511 shares of Class A Common Stock issuable upon conversion of Series A Preferred Stock; and |
| ● | 2,164,238 shares of Class A Common Stock underlying options granted under our 2023 Equity Incentive Plan, exercisable at an average weighted exercise price of $2.32 per share. |
Unless otherwise indicated, all information in this prospectus assumes no exercise of the underwriters’ option to purchase additional securities from us and that no investor elects to purchase pre-funded warrants in lieu of common shares.
6
Investing in our securities involves risk. You should consider the risks, uncertainties and assumptions discussed under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 filed on March 31, 2025 with the Securities and Exchange Commission (“SEC”), which is incorporated herein by reference, and may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. See “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.” The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. If any of these risks were to occur, our business, financial condition, and results of operations could be severely harmed. This could cause the trading price of our common stock to decline, and you could lose all or part of your investment.
7
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, any prospectus supplement and the documents incorporated herein by reference contain forward-looking statements and information within the meaning of Section 27A of the Securities Act of 1933, as amended, or the “Securities Act”, and Section 21E of the Exchange Act, which are subject to the safe harbor created by those sections. These forward-looking statements and information regarding us, our business prospects and our results of operations are subject to certain risks and uncertainties that could cause our actual business, prospects and results of operations to differ materially from those that may be anticipated by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those described under “Risk Factors” herein and in our other filings with the SEC. You should not place undue reliance on these forward-looking statements. You should assume that the information contained in or incorporated by reference in this prospectus, and any prospectus supplement, is accurate only as of the date on the front cover of this prospectus, and any prospectus supplement, or as of the date of the documents incorporated by reference herein or therein, as applicable. We expressly disclaim any intent or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. You are urged to carefully review and consider the various disclosures made by us in this prospectus, any prospectus supplement and the documents incorporated herein by reference and in our other reports filed with the SEC that advise interested parties of the risks and uncertainties that may affect our business.
All statements, other than statements of historical facts, contained in this prospectus, any prospectus supplement and the documents incorporated herein by reference, including statements regarding our plans, objectives and expectations for our business, operations and financial performance and condition, are forward-looking statements. In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “target,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our results, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this prospectus, any prospectus supplement and the documents incorporated herein by reference. Additionally, our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make. Forward-looking statements may include, among other things, statements relating to:
| ● | our financial performance; |
| ● | changes in the market price of our Class A Common Stock; |
| ● | unpredictability in the medical device industry, the regulatory process to approve medical devices, and the clinical development process of our products; |
| ● | the potential need to make design changes to products to meet desired safety and efficacy endpoints; |
| ● | changes in federal or state reimbursement policies that would adversely affect sales of our products; |
8
| ● | introduction of other scientific advancements, including gene therapy or pharmaceuticals, that may impact the need for hearing devices such as cochlear implants or fully implanted active middle ear implants; |
| ● | competition in the medical device industry, and the failure to introduce new products and services in a timely manner or at competitive prices to compete successfully against competitors; |
| ● | disruptions in relationships with our suppliers, or disruptions in our own production capabilities for some of the key components and materials of our products; |
| ● | changes in the need for capital and the availability of financing and capital to fund these needs; |
| ● | changes in interest rates or rates of inflation; |
| ● | legal, regulatory and other proceedings that could be costly and time-consuming to defend; |
| ● | changes in applicable laws or regulations, or changes in how such laws or regulations are applied to us; |
| ● | loss of any of our key intellectual property rights or failure to adequately protect intellectual property rights; |
| ● | our ability to maintain the listing of our securities on the Nasdaq Stock Market; |
| ● | the effects of catastrophic events, including war, terrorism and other international conflicts; and |
| ● | other risks and uncertainties indicated in this prospectus, including those set forth under the section entitled “Risk Factors.” |
Should one or more of these risks or uncertainties materialize, or should any of the underlying assumptions prove incorrect, actual results may vary in material respects from those expressed or implied by these forward-looking statements. Nothing in this prospectus should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on these forward-looking statements. We do not undertake any duty to update these forward-looking statements, except as required by law.
In addition, statements that “we believe”
and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available
to us as of the date of this prospectus, and while we believe such information forms a reasonable basis for such statements, such information
may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or
review of, all relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these
statements.
9
We will receive up to an aggregate of approximately $167.7 million from the exercise of the Warrants, assuming the exercise in full of all of the Warrants for cash. We expect to use the net proceeds, if any, from the exercise of the Warrants for general corporate purposes, which may include acquisitions and other business opportunities. We will have broad discretion over the use of proceeds from the exercise of the Warrants. There is no assurance that the holders of the Warrants will elect to exercise any or all of such Warrants. To the extent that any Public Warrants are exercised on a “cashless basis,” we would not receive any cash proceeds from the exercise of such Public Warrants.
As of the date of this prospectus, the Public Warrants are “out of the money,” which means that the trading price of the shares of our Class A Common Stock underlying our Warrants, which was $ per share on , 2025, is below (i) the $11.50 exercise price (subject to adjustment as described herein) of the Public Warrants. For so long as the Public Warrants remain “out of the money,” we do not expect warrantholders to exercise their warrants and, therefore, we do not expect to receive cash proceeds from any such exercise.
We will not receive any proceeds from the issuance of Class A Common Stock upon conversion of Series A Preferred Stock.
All of the Shortfall Warrants and shares of Class A Common Stock offered by the Selling Securityholders pursuant to this prospectus will be sold by the Selling Securityholders for their respective accounts. We will not receive any of the proceeds from these sales.
10
We are registering the issuance by us of up to an aggregate of 20,964,583 shares of Class A Common Stock, consisting of (i) up to 14,166,666 shares of Class A Common Stock issuable upon the exercise of the Public Warrants by the holders thereof, (ii) up to 3,209,511 shares of Class A Common Stock issuable upon the exercise of 3,209,511 Shortfall Warrants and (iii) up to 3,588,406 shares of Class A Common Stock issuable upon conversion of 4,126,667 shares of Series A Preferred Stock. We are also registering the resale by the Selling Securityholders or their permitted transferees from time to time of up to 3,874,394 Shortfall Warrants and up to 17,718,328 shares of Class A Common Stock, consisting of (i) up to 3,588,406 shares of Class A Common Stock issuable upon conversion of 4,126,667 shares of Series A Preferred Stock, (ii) up to 3,209,511 shares of Class A Common Stock issuable upon the exercise of 3,209,511 Shortfall Warrants and (iii) up to 10,920,411 outstanding shares of Class A Common Stock. We are required to pay all fees and expenses incident to the registration of the Shortfall Warrants and shares of our Class A Common Stock to be offered and sold pursuant to this prospectus. The Selling Securityholders will bear all commissions and discounts, if any, attributable to their sale of Shortfall Warrants and shares of Class A Common Stock.
We will receive proceeds from the Warrants in the event that such Warrants are exercised for cash. We will not receive any of the proceeds from the sale of Shortfall Warrants or shares of Class A Common Stock by the Selling Securityholders. The aggregate proceeds to the Selling Securityholders will be the purchase price of the securities less any discounts and commissions borne by the Selling Securityholders. The term “Selling Securityholder” includes donees, pledgees, transferees or other successors in interest selling securities received after the date of this prospectus from the Selling Securityholders as a gift, pledge, partnership distribution or other transfer. The Selling Securityholders will act independently of us in making decisions with respect to the timing, manner and size of each sale. Such sales may be made on one or more exchanges or in the over-the-counter market or otherwise, at prices and under terms then prevailing or at prices related to the then current market price or in negotiated transactions. The Selling Securityholders may sell their Shortfall Warrants and shares of Class A Common Stock by one or more of, or a combination of, the following methods:
| ● | purchases by a broker-dealer as principal and resale by such broker-dealer for its own account pursuant to this prospectus; |
| ● | ordinary brokerage transactions and transactions in which the broker solicits purchasers; |
| ● | block trades in which the broker-dealer so engaged will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| ● | an over-the-counter distribution in accordance with the rules of Nasdaq; |
| ● | through trading plans entered into by the Selling Securityholders pursuant to Rule 10b5-1 under the Exchange Act, that are in place at the time of an offering pursuant to this prospectus and any applicable prospectus supplement hereto that provide for periodic sales of their securities on the basis of parameters described in such trading plans; |
| ● | to or through underwriters or broker-dealers; |
11
| ● | in “at the market” offerings, as defined in Rule 415 under the Securities Act, at negotiated prices, at prices prevailing at the time of sale or at prices related to such prevailing market prices, including sales made directly on a national securities exchange or sales made through a market maker other than on an exchange or other similar offerings through sales agents; |
| ● | in privately negotiated transactions; |
| ● | in options transactions; |
| ● | through a combination of any of the above methods of sale; or |
| ● | any other method permitted pursuant to applicable law. |
In addition, any shares that qualify for sale pursuant to Rule 144 may be sold under Rule 144 rather than pursuant to this prospectus.
To the extent required, this prospectus may be amended or supplemented from time to time to describe a specific plan of distribution. In connection with distributions of the shares or otherwise, the Selling Securityholders may enter into hedging transactions with broker-dealers or other financial institutions. In connection with such transactions, broker-dealers or other financial institutions may engage in short sales of Shortfall Warrants and shares of Class A Common Stock in the course of hedging the positions they assume with the Selling Securityholders. The Selling Securityholders may also sell Shortfall Warrants and shares of Class A Common Stock short and redeliver the shares to close out such short positions. The Selling Securityholders may also enter into option or other transactions with broker-dealers or other financial institutions which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). The Selling Securityholders may also pledge shares to a broker-dealer or other financial institution, and, upon a default, such broker-dealer or other financial institution, may effect sales of the pledged shares pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Securityholders may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If an applicable prospectus supplement indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by the Selling Securityholders or borrowed from the Selling Securityholders or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from the Selling Securityholders in settlement of those derivatives to close out any related open borrowings of stock. If applicable through securities laws, the third party in such sale transactions will be an underwriter and will be identified in the applicable prospectus supplement (or a post-effective amendment). In addition, the Selling Securityholders may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
In effecting sales, broker-dealers or agents engaged by the Selling Securityholders may arrange for other broker-dealers to participate. Broker-dealers or agents may receive commissions, discounts or concessions from the Selling Securityholders in amounts to be negotiated immediately prior to the sale.
In offering the securities covered by this prospectus, the Selling Securityholders and any broker-dealers who execute sales for the Selling Securityholders may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. Any profits realized by the Selling Securityholders and the compensation of any broker-dealer may be deemed to be underwriting discounts and commissions.
In order to comply with the securities laws of certain states, if applicable, the securities must be sold in such jurisdictions only through registered or licensed brokers or dealers. In addition, in certain states the securities may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
We have advised the Selling Securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of securities in the market and to the activities of the Selling Securityholders and their affiliates. In addition, we will make copies of this prospectus available to the Selling Securityholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Securityholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
At the time a particular offer of securities is made, if required, a prospectus supplement will be distributed that will set forth the number of securities being offered and the terms of the offering, including the name of any underwriter, dealer or agent, the purchase price paid by any underwriter, any discount, commission and other item constituting compensation, any discount, commission or concession allowed or reallowed or paid to any dealer, and the proposed selling price to the public.
A holder of Public Warrants may exercise its Public Warrants in accordance with the Warrant Agreement, on or before the expiration date set forth therein by surrendering, at the office of the Warrant Agent, the certificate evidencing such Public Warrant, with the form of election to purchase set forth thereon, properly completed and duly executed, accompanied by full payment of the exercise price and any and all applicable taxes due in connection with the exercise of a Public Warrant, subject to any applicable provisions relating to cashless exercises in accordance with the Warrant Agreement.
12
The Selling Securityholders may from time to time offer and sell any or all of the Shortfall Warrants and shares of Class A Common Stock set forth below pursuant to this prospectus. When we refer to the “Selling Securityholders” in this prospectus, we mean the persons listed in the table below, and the pledgees, donees, transferees, assignees, successors and others who later come to hold any of the Selling Securityholders’ interest in the Shortfall Warrants or shares of Class A Common Stock after the date of this prospectus such that registration rights shall apply to those securities.
Pursuant to agreements with the Selling Securityholders, we agreed to file a registration statement with the SEC for the purposes of registering the Shortfall Warrants and Class A Common Stock for resale. The shares of Class A Common Stock being offered by the Selling Securityholders consist of (i) up to 3,588,406 shares of Class A Common Stock issuable upon the conversion of 4,126,667 shares of Series A Preferred Stock pursuant to the Certificate of Designation, (ii) up to 3,209,511 shares of Class A Common Stock issuable upon the exercise of 3,209,511 Shortfall Warrants and (iii) up to 10,920,411 outstanding shares of Class A Common Stock.
Given the current market price of our Class A Common Stock, certain of the Selling Securityholders who paid less for their shares than such current market price will receive a higher rate of return on any such sales than the public securityholders who purchased their Class A Common Stock in the IPO or any Selling Securityholder who paid more for their shares than the current market price.
The following tables are prepared based on information provided to us by the Selling Securityholders. It sets forth the name of each Selling Securityholder, the aggregate number of Shortfall Warrants and shares of Class A Common Stock that each Selling Securityholder may offer pursuant to this prospectus, and the beneficial ownership of each Selling Securityholder both before and after the offering. We have based percentage ownership prior to this offering on 21,520,932 shares of Class A Common Stock outstanding as of July 25, 2025. In calculating percentages of shares of Class A Common Stock owned by a particular Selling Securityholder, we treated as outstanding the number of shares of our Class A Common Stock issuable upon exercise of that particular Selling Securityholder’s Shortfall Warrants and conversion of that particular Selling Securityholder’s Series A Preferred Stock, if any, and did not assume the exercise of any other Selling Securityholders’ Shortfall Warrants or conversion of any other Selling Securityholders’ Series A Preferred Stock.
13
We cannot advise you as to whether the Selling Securityholders will in fact sell any or all of such Shortfall Warrants or shares of Class A Common Stock. In addition, the Selling Securityholders may sell, transfer or otherwise dispose of, at any time and from time to time, the Shortfall Warrants or Class A Common Stock in transactions exempt from the registration requirements of the Securities Act after the date of this prospectus, subject to the restrictions on transfer described above. For purposes of this table, we have assumed that the Selling Securityholders will have sold all of the securities covered by this prospectus upon the completion of the offering.
| Beneficial Ownership Before the Offering |
Securities to be Sold in the Offering |
Beneficial Ownership After the Offering |
||||||||||||||||||||||||||||||||||
| Name of Selling Securityholder | Shares of Class A Common Stock |
% | Shortfall Warrants |
Shares of Class A Common Stock |
% | Shortfall Warrants |
Shares of Class A Common Stock |
% | Shortfall Warrants |
|||||||||||||||||||||||||||
| Glen A. Taylor(1) | 13,909,614 | 55.3 | % | — | 11,159,614 | 44.4 | % | — | 2,750,000 | 10.9 | % | — | ||||||||||||||||||||||||
| Anzu SPAC GP I LLC(2) | 2,718,841 | 11.2 | % | — | 2,718,841 | 11.2 | % | — | 0 | * | — | |||||||||||||||||||||||||
| Brent T. Lucas(3) | 1,116,081 | 4.9 | % | — | 118,958 | * | — | 997,123 | 4.4 | % | — | |||||||||||||||||||||||||
| Allen Lenzmeier(4) | 163,642 | * | — | 163,642 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Paul Waldon(5) | 222,762 | * | — | 222,762 | * | — | * | — | ||||||||||||||||||||||||||||
| Daniel J. Hirsch(6) | 25,000 | * | — | 25,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Priya Cherian Huskins(7) | 25,000 | * | — | 25,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Susan J. Kantor(8) | 140,387 | * | — | 25,000 | * | — | 115,387 | * | — | |||||||||||||||||||||||||||
| Diane L. Dewbrey(9) | 25,000 | * | — | 25,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Teresa A. Harris(10) | 25,000 | * | — | 25,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Arena Capital Advisors(11) | 45,000 | * | — | 45,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Entities Affiliated with Fir Tree Capital Management, LP(12) | 125,000 | * | — | 125,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Entities Affiliated with Sandia Investment Management LP(13) | 100,000 | * | — | 100,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Entities Affiliated with Weiss Asset Management LP(14) | 20,000 | * | — | 20,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| SZOP Multistrat LP(15) | 20,000 | * | — | 20,000 | * | — | 0 | * | — | |||||||||||||||||||||||||||
| Polar Multi-Strategy Master Fund(16) | 648,771 | 3.3 | % | — | 80,000 | * | — | 568,771 | 2.9 | % | — | |||||||||||||||||||||||||
| Entities Affiliated with Meteora Capital, LLC(17) | 3,743,629 | 9.99 | % | 3,209,511 | 3,743,629 | 9.9 | % | 3,209,511 | 0 | * | — | |||||||||||||||||||||||||
| * | Less than one percent. |
| (1) | Includes (i) 2,953,607 shares of Class A Common Stock held by Mr. Taylor directly, (ii) 2,526,058 shares of Class A Common Stock held by Taylor Sports Group of which Mr. Taylor is the owner and chairman, (iii) 4,810,384 shares of Class A Common Stock held by GAT Funding, LLC, which Mr. Taylor controls and (iv) 869,565 shares of Class A Common Stock issuable upon the conversion of 1,000,000 shares of Series A Preferred Stock, which were issued to GAT Funding, LLC pursuant to the Legacy Envoy Medical Bridge Note. Mr. Taylor served as a member of the Legacy Envoy Board prior to the Business Combination and now serves as a member of the board of directors of the Company. |
| (2) | Includes (i) 1,849,276 shares of Class A Common Stock issuable upon the conversion of 2,126,667 shares of Series A Preferred Stock received by Anzu GP in a private exchange offer for shares of Anzu Class B Common Stock and (ii) 869,565 shares of Class A Common Stock issuable upon the conversion of an aggregate of 1,000,000 shares of Series A Preferred Stock, which were issued to AICP III L.P., Anzu Industrial Capital Partners III, L.P. and Anzu Industrial Capital Partners III QP, L.P., each an affiliate of Anzu GP. Whitney Haring-Smith, David Seldin and David Michael share voting and investment control over shares held by Anzu GP by virtue of their shared control of Anzu GP. The business address of Anzu GP is 12610 Race Track Road, Suite 250 Tampa, FL 33626. |
| (3) | Includes (i) 200,780 shares of Class A Common Stock held directly by Mr. Lucas, (ii) 800,327 shares of Class A Common Stock issuable upon exercise of stock options, (iii) 110,987 shares of Class A Common Stock issuable upon exercise of Public Warrants, (iv) 1,972 shares of Class A Common Stock held by Mr. Lucas’s spouse, (v) 5,991 shares of Class A Common Stock held by the Brent T. Lucas Irrevocable Trust of which Mr. Lucas is a beneficiary, and (vi) 12,720 shares of Class A Common Stock held by the Brent T. Lucas Family Education Trust of which Mr. Lucas’ children are beneficiaries and Mr. Lucas is a trustee. |
| (4) | Includes (i) 159,190 shares of Class A Common Stock held by Allen U. Lenzmeier and Kathleen Lenzmeier, Trustees of the Allen U. Lenzmeier Revocable Trust U/A dtd 11/29/2012 and (ii) 4,452 shares of Class A Common Stock held by Allen Lenzmeier and Kathleen Lenzmeier, JTWROS. Allen Lenzmeier previously served as a member of the Legacy Envoy Board. |
14
| (5) | Includes (i) 1,170 shares of Class A Common Stock held directly by Paul Waldon and (ii) 221,592 shares of Class A Common Stock held by Carefree Capital, Inc. Mr. Waldon previously served as a member of the Legacy Envoy Board. |
| (6) | Daniel J. Hirsch previously served as a member of the board of directors and as Chief Financial Officer and Corporate Secretary of Anzu. |
| (7) | Priya Cherian Huskins previously served as a member of the board of directors of Anzu. |
| (8) | Susan J. Kantor previously served as a member of the board of directors of Anzu prior to the Business Combination and now serves as a member of the board of directors of the Company. Includes stock options to purchase 62,5000 shares of Class A Common Stock. |
| (9) | Diane L. Dewbrey previously served as a member of the board of directors of Anzu. |
| (10) | Teresa A. Harris previously served as a member of the board of directors of Anzu. |
| (11) | Arena Capital Advisors, LLC is the general partner of Arena Capital Advisors and may be deemed to have voting control and investment discretion over the securities. The partners of Arena Capital Advisors, LLC are Daniel Elperin, Jeremy Sagi and Sanije Perrett, and as such Mr. Elperin, Mr. Sagi and Mr. Perrett may be deemed to beneficially own the securities. |
| (12) | Includes (i) 15,217 shares of Class A Common Stock held directly by Fir Tree Capital Opportunity Master Fund, LP, (ii) 22,997 shares of Class A Common Stock held directly by Fir Tree Capital Opportunity Master Fund III, LP, (iii) 32,087 shares of Class A Common Stock held directly by FT SOF XIII (SPAC) Holdings, LLC, (iv) 24,226 shares of Class A Common Stock held directly by Fir Tree Value Master Fund, LP and (v) 30,473 shares of Class A Common Stock held directly by Boston Patriot Merrimack St LLC (collectively, the “Fir Tree Funds”). Fir Tree Capital Management, LP is the Investment Manager of the Fir Tree Funds and has sole investment and dispositive power over the shares held thereby. Clinton Biondo and David Sultan are Managing Partners of Fir Tree Capital Management, LP and may be deemed to have voting and investment control with respect to the shares held by these entities. Each of the parties in this footnote disclaims any beneficial ownership of the reported shares other than to the extent of any pecuniary interest the party may have therein. |
| (13) | Includes shares of Class A Common Stock allocated to investors managed by Sandia Investment Management LP (“Sandia”). Tim Sichler serves as Founder & CIO of the general partner of Sandia, and in such capacity may be deemed to be the beneficial owner having shared voting power and shared investment power over the securities described in this footnote. Each of the parties in this footnote disclaims any beneficial ownership of the reported shares other than to the extent of any pecuniary interest the party may have therein. The business address of these entities and Mr. Sichler is 201 Washington Street, Boston, Massachusetts 02108. |
| (14) | Includes (i) 7,400 shares of Class A Common Stock held directly by Brookdale Global Opportunity Fund (“BGO”) and (ii) 12,600 shares of Class A Common Stock held directly by Brookdale International Partners, L.P. (“BIP”). Andrew Weiss is the Manager of WAM GP LLC, which is the general partner of Weiss Asset Management LP, the investment manager of BGO and BIP. WAM GP LLC is also the Manager of BIP GP LLC, the general partner of BIP. Mr. Weiss has voting and dispositive power with respect to the securities held by the BGO and BIP. Mr. Weiss, WAM GP LLC, Weiss Asset Management LP and BIP GP LLC each disclaim beneficial ownership of the shares held by BGO and BIP, except to the extent of their respective pecuniary interests therein. |
| (15) | SZOP Multistrat Management LLC (the “SZOP Manager”) serves as the investment manager to SZOP Multistrat LP. Antonio Ruiz-Gimenez and Kerry Propper serve as managing members of the SZOP Manager (all of the foregoing, collectively, the “SZOP Reporting Persons”). The SZOP Reporting Persons may be deemed to have shared voting and dispositive power with respect to the shares of Class A Common Stock owned by SZOP Multistrat LP. |
| (16) | Polar Multi-Strategy Master Fund (the “Polar Fund”) is under management by Polar Asset Management Partners Inc. (“PAMPI”). PAMPI serves as Investment Advisor to the Polar Fund and has control and discretion over the securities held by the Polar Fund. As such, PAMPI may be deemed the beneficial owner of the securities held by the Polar Fund. PAMPI disclaims any beneficial ownership of the reported securities other than to the extent of any pecuniary interest therein. The ultimate natural persons who have voting and dispositive power over the securities held by the Polar Fund are Paul Sabourin and Abdalla Ruken, Co-Chief Investment Officers of PAMPI. |
| (17) | Includes (i) 28,416 shares of Class A Common Stock held directly by Meteora Capital Partners, LP, (ii) 20,425 shares of Class A Common Stock held directly by Meteora Select Trading Opportunities Master, LP, (iii) 9,453 shares of Class A Common Stock held directly by Meteora Special Opportunity Fund I, LP, (iv) 1,030 shares of Class A Common Stock held directly by Meteora Strategic Capital, LLC, (v) 3,874,394 shares of Class A Common Stock issuable upon the exercise of the Shortfall Warrants held directly by the Meteora FPA Parties, (vi) 26,142 shares of Class A Common Stock held directly by Boothbay Absolute Return Strategies, LP and (vii) 23,046 shares of Class A Common Stock held directly by Boothbay Diversified Alpha Master Fund, LP (collectively, the “Meteora Funds”). Meteora Capital, LLC serves as investment manager to the Meteora Funds. Vik Mittal serves as the Managing Member of Meteora Capital, LLC. (collectively, the “Meteora Funds”). Meteora Capital, LLC serves as investment manager to the Meteora Funds. Vik Mittal serves as the Managing Member of Meteora Capital, LLC. The Meteora FPA Parties are subject to a blocker which prevents them from exercising their Shortfall Warrants to the extent that, upon such exercise, the Meteora Funds would collectively beneficially own in excess of 9.99% of shares of Class A Common Stock outstanding as a result of the exercise. |
15
DESCRIPTION OF PRIVATE PLACEMENTS
Description of 2023 PIPE Private Placements
The following description is qualified in its entirety by the terms and conditions of the Subscription Agreement and Convertible Promissory Note, which are included as Exhibit 10.1 and Exhibit 10.2 hereto, respectively, and incorporated by reference into the registration statement of which this prospectus forms a part. The following description may not contain all the information with respect to the Subscription Agreement and Convertible Promissory Note that is important to you. We encourage you to read the Subscription Agreement and Convertible Promissory Note in their entirety.
Pursuant to the Subscription Agreement, dated April 17, 2023 (as amended to date, the “Subscription Agreement”), by and between Anzu and Sponsor, the Company issued, and certain affiliates of the Sponsor purchased, concurrently with the Closing, an aggregate of 1,000,000 shares of Series A Preferred Stock in a private placement at a price of $10.00 per share for an aggregate purchase price of $10,000,000.
Pursuant to the Convertible Promissory Note, dated April 17, 2023, between Legacy Envoy and GAT, the Company issued 1,000,000 shares of Series A Preferred Stock to GAT Funding, LLC concurrently with the Closing.
Registration Rights
We are required to maintain the effectiveness of the registration statement of which this prospectus forms a part until the shares Class A Common Stock issued upon conversion of the shares of Series A Preferred Stock issued in the 2023 PIPE Private Placement are sold.
Description of 2023 Warrant Private Placement
The following description is qualified in its entirety by the terms and conditions of the Forward Purchase Agreement, which is included as Exhibit 10.3 hereto and incorporated by reference into the registration statement of which this prospectus forms a part. The following description may not contain all the information with respect to the Forward Purchase Agreement that is important to you. We encourage you to read the Forward Purchase Agreement in their entirety.
Pursuant to the terms of the Forward Purchase Agreement, dated April 17, 2023 (as amended to date, the “Forward Purchase Agreement”), by and between Anzu, Legacy Envoy, and the Meteora FPA Parties Sponsor, the Company issued 3,874,394 Shortfall Warrants, of which 3,209,511 remaining outstanding. The currently outstanding Shortfall Warrants are exercisable for 3,209,511 shares of Class A Common Stock at an exercise price of $1.50 per share, subject to adjustment.
Pursuant to the Convertible Promissory Note, dated April 17, 2023, between Legacy Envoy and GAT, the Company issued 1,000,000 shares of Series A Preferred Stock to GAT Funding, LLC concurrently with the Closing.
Registration Rights
We are required to maintain the effectiveness of the registration statement of which this prospectus forms a part until the shares Class A Common Stock issued upon exercise of the Shortfall Warrants are sold.
16
The following summary of the material terms of our securities is not intended to be a complete summary of the rights and preferences of such securities, and is qualified by reference to our Certificate of Incorporation, our Bylaws and the Warrant-related documents described herein, which are exhibits to the registration statement of which this prospectus is a part. We urge you to read each of our Certificate of Incorporation, the Bylaws and the Warrant-related documents described herein in their entirety for a complete description of the rights and preferences of our securities.
Authorized and Outstanding Stock
Our Certificate of Incorporation authorizes the issuance of 500,000,000 shares of the Company, consisting of 400,000,000 shares of Class A Common Stock, par value $0.0001 per share and 100,000,000 shares of preferred stock, $0.0001 par value per share. As of the date of this prospectus, there were 21,520,932 shares of Class A Common Stock and 4,126,667 shares of Series A Preferred Stock outstanding. The outstanding shares of Common Stock are duly authorized, validly issued, fully paid and non-assessable.
Common Stock
Holders of our Class A Common Stock will be entitled to one vote for each share held as of the applicable record date on all matters properly submitted to a vote of stockholders, including the election or removal of directors. Unless specified in our governing documents, or as required by applicable provisions of the DGCL or applicable stock exchange rules, the affirmative vote of a plurality of the votes cast at any meeting of the stockholders at which there is a quorum by the stockholders present in person or represented by proxy at the meeting and entitled to vote thereon will be required to approve any such matter voted on by stockholders. The Board is divided into three classes, each of which will generally serve for a term of three (3) years with only one (1) class of directors being elected each year. Our stockholders will not have cumulative voting rights in the election of directors.
Authorized Common Stock
The Company is authorized under the Certificate of Incorporation to issue 400,000,000 shares of Class A Common Stock. The outstanding shares of Class A Common Stock are fully paid and nonassessable.
Voting Rights
Holders of our Class A Common Stock will be entitled to one vote for each share held as of the applicable record date on all matters properly submitted to a vote of stockholders, including the election or removal of directors. Unless specified in our governing documents, or as required by applicable provisions of the General Corporation Law of the State of Delaware (the “DGCL”) or applicable stock exchange rules, the affirmative vote of a plurality of the votes cast at any meeting of the stockholders at which there is a quorum by the stockholders present in person or represented by proxy at the meeting and entitled to vote thereon will be required to approve any such matter voted on by stockholders. The Board is divided into three (3) classes, each of which will generally serve for a term of three (3) years with only one (1) class of directors being elected each year. Our stockholders will not have cumulative voting rights in the election of directors.
Dividend Rights
Holders of Class A Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Board of Directors, in its discretion, out of funds legally available therefor, subject to any preferential dividend rights of outstanding preferred stock.
Liquidation Rights
In the event of a liquidation, dissolution or winding up of the Company, the holders of the Company’s Class A Common Stock are entitled to share ratably in all assets remaining after the payment of all of the Company’s liabilities and subject to the liquidation preferences of any outstanding preferred stock.
Other Rights and Preferences
The Class A Common Stock does not carry preemptive rights, is not redeemable, does not have any conversion rights, is not subject to further calls and is not subject to any sinking fund provisions. The rights and preferences of holders of the Class A Common Stock are subject to the rights of any series of preferred stock that the Company may issue.
17
Preferred Stock
Our Certificate of Incorporation authorizes the issuance of up to 100,000,000 shares of preferred stock and the Board by resolution or resolutions, to the maximum extent permitted by law, to fix by resolution or resolutions the designation, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any series of preferred stock, including, without limitation, authority to fix by resolution or resolutions the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices, and liquidation preferences of any such series, and the number of shares constituting any such series and the designation thereof, or any of the foregoing.
Pursuant to a Certificate of Designations filed with the Secretary of State of the State of Delaware on September 29, 2023, we have designated 10,000,000 shares of our authorized but unissued shares of preferred stock as Series A Preferred Stock.
If we offer a specific class or series of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such offering and will file a copy of the certificate establishing the terms of the preferred stock with the SEC. The preferred stock offered by this prospectus, when issued, will not have, or be subject to, any preemptive or similar rights.
Transfer Agent and Registrar
The transfer agent and registrar for any series or class of preferred stock will be set forth in each applicable prospectus supplement.
Shortfall Warrants
Each whole Shortfall Warrant represents the right to purchase one share of Class A Common Stock at a price of $1.50 per share, subject to adjustment as described below. The Shortfall Warrants do not include any redemption features and do not provide for cashless exercise or net share settlement. The Shortfall Warrants will expire on December 31, 2025 at 5:00 p.m., New York City time. The Shortfall Warrants are exercisable at any time until the expiration thereof, except that the Shortfall Warrants cannot be exercised by a holder thereof if, after giving effect thereto, such holder would beneficially own more than 9.9% of the outstanding shares of Class A Common Stock immediately after giving effect to the issuance of shares of Class A Common Stock issuable upon exercise of such Shortfall Warrants (the “Maximum Percentage”), which Maximum Percentage may be increased or decreased by the holders thereof, upon written notice to the Company, to any specified percentage not in excess of 9.9%.
The exercise price of the Shortfall Warrants will be adjusted on the first scheduled trading day of each week (each a “Reset Date”) commencing with the first week following the thirtieth day after the Closing to the volume weighted average price (“VWAP Price”) of the shares of Class A Common Stock of the week immediately prior to the applicable Reset Date, but not lower than $1.50; provided, however, that the Reset Price may be further reduced to the price at which the Company sells, issues or grants any shares of Class A Common Stock or securities convertible or exchangeable into shares of Class A Common Stock (other than as otherwise contemplated by a business combination agreement relating to the Business Combination). In addition, the exercise price of the Shortfall Warrants is subject to certain customary adjustments in the event of certain events affecting the price of our Class A Common Stock, such as stock splits and combinations, or the distribution of options, rights or warrants.
A holder of the Shortfall Warrants does not have the rights or privileges of holders of Class A Common Stock or have any voting rights with respect to the Shortfall Warrants or the underlying shares of Class A Common Stock until it exercises the Shortfall Warrant and receives shares of Class A Common Stock. After the issuance of shares of Class A Common Stock underlying the Shortfall Warrants, a holder will be entitled to one vote for each share of Class A Common Stock held of record on all matters to be voted on by stockholders.
Each Shortfall Warrant may be exercised in part or in full. If we fail for any reason to deliver to the holder the applicable shares before the required date, we must pay certain liquidated damages to the holder. The form of Shortfall Warrant is attached as Exhibit 4.2 to the registration statement of which this prospectus forms a part.
Public Warrants
Each whole Public Warrant entitles the registered holder to purchase one whole share of Class A Common Stock at a price of $11.50 per share, subject to adjustment as discussed below, at any time commencing on October 29, 2023. Pursuant to the Warrant Agreement, a holder of Public Warrants may exercise its Public Warrants only for a whole number of shares of Class A Common Stock. This means that only a whole Public Warrant may be exercised at any given time by a Public Warrant holder. The Public Warrants will expire on September 29, 2028, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
18
The Company will not be obligated to deliver any shares of Class A Common Stock pursuant to the exercise of a Public Warrant and will have no obligation to settle such Public Warrant exercise unless a registration statement under the Securities Act with respect to the shares of Class A Common Stock underlying the Public Warrants is then effective and a prospectus relating thereto is current, subject to the Company satisfying its obligations described below with respect to registration. No Public Warrant will be exercisable and the Company will not be obligated to issue shares of Class A Common Stock upon exercise of a Public Warrant unless Class A Common Stock issuable upon such Public Warrant exercise has been registered, qualified or deemed to be exempt under the securities laws of the state of residence of the registered holder of the Public Warrants. In the event that the conditions in the two immediately preceding sentences are not satisfied with respect to a Public Warrant, the holder of such Public Warrant will not be entitled to exercise such Public Warrant and such Public Warrant may have no value and expire worthless. In no event will the Company be required to net cash settle any Public Warrant. The Company will be obligated to file as soon as practicable, but in no event later than 15 business days after the Closing, and to use its best efforts to file with the SEC a registration statement covering the shares of Class A Common Stock issuable upon exercise of the Public Warrants, to cause such registration statement to become effective and to maintain a current prospectus relating to those shares of Class A Common Stock until the Public Warrants expire or are redeemed, as specified in the Warrant Agreement. If a registration statement covering the shares of Class A Common Stock issuable upon exercise of the Public Warrants is not effective by the 60th business day after the Closing (December 29, 2023), Public Warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise Public Warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. If that exemption, or another exemption, is not available, holders will not be able to exercise their Public Warrants on a cashless basis. In no event will the Company be required to net cash settle any Public Warrant.
Once the registration statement of which this prospectus forms a part is declared effective by the SEC, the Public Warrants will become exercisable and Public Warrant holders will be able to provide their instructions to their broker-dealers (DTC participants) to exercise such Public Warrants as provided in the Warrant Agreement.
Redemption of Public Warrants When the Price Per Share of Class A Common Stock Equals or Exceeds $18.00
Once the Public Warrants become exercisable, under certain conditions, the Company may call the Public Warrants for redemption:
| ● | In whole and not in part; |
| ● | At a price of $0.01 per Public Warrant; |
| ● | Upon not less than 30 days’ prior written notice of redemption to each Public Warrant holder; and |
| ● | If, and only if, the last reported the sale price of the Class A Common Stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three trading days before the Company sends the notice of redemption to the Public Warrant holders. |
We will not redeem the warrants as described above unless a registration statement under the Securities Act covering the issuance of the shares of Class A Common Stock issuable upon exercise of the Public Warrants is then effective and a current prospectus relating to those shares of Class A Common Stock is available throughout the 30 consecutive day redemption period. If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Redemption of Public Warrants When the Price Per Share of Class A Common Stock Equals or Exceeds $10.00
Once the Public Warrants become exercisable, we may redeem the outstanding Public Warrants:
| ● | in whole and not in part; |
| ● | at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares determined by reference to the table below, based on the redemption date and the “fair market value” of the Class A Common Stock (as defined below) except as otherwise described below; |
| ● | if, and only if, the closing price of the Class A Common Stock equals or exceeds $10.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any period of 30 consecutive trading days ending three trading days before we send the notice of redemption to the Public Warrant holders; and |
| ● | if the closing price of the Class A Common Stock for any 20 trading days within any period of 30 consecutive trading days ending on the third trading day prior to the date on which we send the notice of redemption to the Public Warrant holders is less than $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like). |
19
Beginning on the date the notice of redemption is given until the warrants are redeemed or exercised, holders may elect to exercise their warrants on a cashless basis. The numbers in the table below represent the number of shares of Class A Common Stock that a warrant holder will receive upon such cashless exercise in connection with a redemption by the Company pursuant to this redemption feature, based on the “fair market value” of Class A Common Stock on the corresponding redemption date (assuming holders elect to exercise their warrants and such warrants are not redeemed for $0.10 per warrant), determined for these purposes based on volume weighted average price of Class A Common Stock during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of warrants, and the number of months that the corresponding redemption date precedes the expiration date of the warrants, each as set forth in the table below. The Company will provide its warrant holders with the final fair market value no later than one business day after the 10-trading day period described above ends. The share prices set forth in the column headings of the table below will be adjusted as of any date on which the number of shares issuable upon exercise of a warrant or the exercise price of a warrant is adjusted as set forth under the heading “Anti-dilution Adjustments” below.
| Redemption Date (period to expiration of |
Fair Market Value of Class A Common Stock | |||||||||||||||||||||||||||||||||||
| Public Warrants) | <$10.00 | $11.00 | $12.00 | $13.00 | $14.00 | $15.00 | $16.00 | $17.00 | >$18.00 | |||||||||||||||||||||||||||
| 60 months | 0.261 | 0.281 | 0.297 | 0.311 | 0.324 | 0.337 | 0.348 | 0.358 | 0.361 | |||||||||||||||||||||||||||
| 57 months | 0.257 | 0.277 | 0.294 | 0.310 | 0.324 | 0.337 | 0.348 | 0.358 | 0.361 | |||||||||||||||||||||||||||
| 54 months | 0.252 | 0.272 | 0.291 | 0.307 | 0.322 | 0.335 | 0.347 | 0.357 | 0.361 | |||||||||||||||||||||||||||
| 51 months | 0.246 | 0.268 | 0.287 | 0.304 | 0.320 | 0.333 | 0.346 | 0.357 | 0.361 | |||||||||||||||||||||||||||
| 48 months | 0.241 | 0.263 | 0.283 | 0.301 | 0.317 | 0.332 | 0.344 | 0.356 | 0.361 | |||||||||||||||||||||||||||
| 45 months | 0.235 | 0.258 | 0.279 | 0.298 | 0.315 | 0.330 | 0.343 | 0.356 | 0.361 | |||||||||||||||||||||||||||
| 42 months | 0.228 | 0.252 | 0.274 | 0.294 | 0.312 | 0.328 | 0.342 | 0.355 | 0.361 | |||||||||||||||||||||||||||
| 39 months | 0.221 | 0.246 | 0.269 | 0.290 | 0.309 | 0.325 | 0.340 | 0.354 | 0.361 | |||||||||||||||||||||||||||
| 36 months | 0.213 | 0.239 | 0.263 | 0.285 | 0.305 | 0.323 | 0.339 | 0.353 | 0.361 | |||||||||||||||||||||||||||
| 33 months | 0.205 | 0.232 | 0.257 | 0.280 | 0.301 | 0.320 | 0.337 | 0.352 | 0.361 | |||||||||||||||||||||||||||
| 30 months | 0.196 | 0.224 | 0.250 | 0.274 | 0.297 | 0.316 | 0.335 | 0.351 | 0.361 | |||||||||||||||||||||||||||
| 27 months | 0.185 | 0.214 | 0.242 | 0.268 | 0.291 | 0.313 | 0.332 | 0.350 | 0.361 | |||||||||||||||||||||||||||
| 24 months | 0.173 | 0.204 | 0.233 | 0.260 | 0.285 | 0.308 | 0.329 | 0.348 | 0.361 | |||||||||||||||||||||||||||
| 21 months | 0.161 | 0.193 | 0.223 | 0.252 | 0.279 | 0.304 | 0.326 | 0.347 | 0.361 | |||||||||||||||||||||||||||
| 18 months | 0.146 | 0.179 | 0.211 | 0.242 | 0.271 | 0.298 | 0.322 | 0.345 | 0.361 | |||||||||||||||||||||||||||
| 15 months | 0.130 | 0.164 | 0.197 | 0.230 | 0.262 | 0.291 | 0.317 | 0.342 | 0.361 | |||||||||||||||||||||||||||
| 12 months | 0.111 | 0.146 | 0.181 | 0.216 | 0.250 | 0.282 | 0.312 | 0.339 | 0.361 | |||||||||||||||||||||||||||
| 9 months | 0.090 | 0.125 | 0.162 | 0.199 | 0.237 | 0.272 | 0.305 | 0.336 | 0.361 | |||||||||||||||||||||||||||
| 6 months | 0.065 | 0.099 | 0.137 | 0.178 | 0.219 | 0.259 | 0.296 | 0.331 | 0.361 | |||||||||||||||||||||||||||
| 3 months | 0.034 | 0.065 | 0.104 | 0.150 | 0.197 | 0.243 | 0.286 | 0.326 | 0.361 | |||||||||||||||||||||||||||
| 0 months | — | — | 0.042 | |||||||||||||||||||||||||||||||||
The “fair market value” of our Class A Common Stock shall mean the average last reported sale price of our Class A Common Stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of Public Warrants.
The exact fair market value and redemption date may not be set forth in the table above, in which case, if the fair market value is between two values in the table or the redemption date is between two redemption dates in the table, the number of shares of Class A Common Stock to be issued for each Public Warrant redeemed will be determined by a straight-line interpolation between the number of shares set forth for the higher and lower fair market values and the earlier and later redemption dates, as applicable, based on a 365- or 366-day year, as applicable. In no event will the warrants be exercisable on a cashless basis in connection with this redemption feature for more than 0.361 shares of Class A Common Stock per Public Warrant (subject to adjustment). Finally, as reflected in the table above, if the Public Warrants are out of the money and about to expire, they cannot be exercised on a cashless basis in connection with a redemption by us pursuant to this redemption feature, since they will not be exercisable for any shares of Class A Common Stock. At such time as the warrants become exercisable for Class A Common Stock, we will use commercially reasonable efforts to register under the Securities Act the security issuable upon the exercise of the Public Warrants.
Redemption Procedures
A holder of a Public Warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such Public Warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the Warrant Agent’s actual knowledge, would beneficially own in excess of 9.8% (or such other amount as a holder may specify) of the shares of Class A Common Stock issued and outstanding immediately after giving effect to such exercise.
20
Anti-Dilution Adjustments
If the number of outstanding shares of Class A Common Stock is increased by a stock dividend payable in shares of Class A Common Stock, or by a split-up of shares of Class A Common Stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of Class A Common Stock issuable on exercise of each Public Warrant will be increased in proportion to such increase in the outstanding shares of Class A Common Stock. A rights offering to holders of Class A Common Stock entitling holders to purchase shares of Class A Common Stock at a price less than the fair market value will be deemed a stock dividend of a number of shares of Class A Common Stock equal to the product of (i) the number of shares of Class A Common Stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for Class A Common Stock) and (ii) one minus the quotient of (x) the price per share of Class A Common Stock paid in such rights offering divided by (y) the fair market value. For these purposes (i) if the rights offering is for securities convertible into or exercisable for Class A Common Stock, in determining the price payable for Class A Common Stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) fair market value means the volume weighted average price of Class A Common Stock as reported during the 10 trading day period ending on the trading day prior to the first date on which the shares of Class A Common Stock trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if the Company, at any time while the Public Warrants are outstanding and unexpired, pays a dividend or makes a distribution in cash, securities or other assets to the holders of Class A Common Stock on account of such shares of Class A Common Stock (or other shares of our capital stock into which the Public Warrants are convertible), other than (i) as described above, (ii) certain ordinary cash dividends (initially defined as up to $0.50 per share in a 365 day period), (iii) to satisfy the redemption rights of the holders of Class A Common Stock in connection with the Closing, or (iv) to satisfy the redemption rights of the holders of Class A Common Stock in connection with a stockholder vote to amend the Certificate of Incorporation with respect to any provision relating to stockholders’ rights, then the Public Warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of Class A Common Stock in respect of such event.
If the number of outstanding shares of our Class A Common Stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of Class A Common Stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of Class A Common Stock issuable on exercise of each Public Warrant will be decreased in proportion to such decrease in outstanding shares of Class A Common Stock.
Whenever the number of shares of Class A Common Stock purchasable upon the exercise of the Public Warrants is adjusted, as described above, the Public Warrant exercise price will be adjusted by multiplying the Public Warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of Class A Common Stock purchasable upon the exercise of the Public Warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of Class A Common Stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding shares of Class A Common Stock (other than those described above or that solely affects the par value of such shares of Class A Common Stock), or in the case of any merger or consolidation of the Company with or into another corporation (other than a consolidation or merger in which the Company is the continuing corporation and that does not result in any reclassification or reorganization of outstanding shares of Class A Common Stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of the Company as an entirety or substantially as an entirety in connection with which the Company is dissolved, the holders of the Public Warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the Public Warrants and in lieu of the shares of Class A Common Stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the Public Warrants would have received if such holder had exercised their Public Warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of Class A Common Stock in such a transaction is payable in the form of Class A Common Stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Public Warrant properly exercises the Public Warrant within 30 days following public disclosure of such transaction, the Public Warrant exercise price will be reduced as specified in the Warrant Agreement based on the Black-Scholes value (as defined in the Warrant Agreement) of the Public Warrant. The purpose of such exercise price reduction is to provide additional value to holders of the Public Warrants when an extraordinary transaction occurs during the exercise period of the Public Warrants pursuant to which the holders of the Public Warrants otherwise do not receive the full potential value of the Public Warrants in order to determine and realize the option value component of the Public Warrant. This formula is to compensate the Public Warrant holder for the loss of the option value portion of the Public Warrant due to the requirement that the Public Warrant holder exercise the Public Warrant within 30 days of the event. The Black-Scholes model is an accepted pricing model for estimating fair market value where no quoted market price for an instrument is available.
21
The Public Warrants have been issued in registered form under the Warrant Agreement. The Warrant Agreement provides that the terms of the Public Warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 65% of the then outstanding Public Warrants to make any change that adversely affects the interests of the registered holders of Public Warrants.
The Public Warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the Warrant Agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to the Company, for the number of Public Warrants being exercised. The Public Warrant holders do not have the rights or privileges of holders of Class A Common Stock and any voting rights until they exercise their Public Warrants and receive shares of Class A Common Stock. After the issuance of shares of Class A Common Stock upon exercise of the Public Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No fractional shares will be issued upon exercise of the Public Warrants. If, upon exercise of the Public Warrants, a holder would be entitled to receive a fractional interest in a share, the Company will, upon exercise, round down to the nearest whole number of shares of Class A Common Stock to be issued to the Public Warrant holder.
Certain Anti-Takeover Provisions of the Certificate of Incorporation and Bylaws
The Certificate of Incorporation, Bylaws and the DGCL contain provisions as summarized in the following paragraphs that are intended to enhance the likelihood of continuity and stability in the composition of the Board. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of the Board to maximize stockholder value in connection with any unsolicited offer to acquire the Company. However, these provisions may have an anti-takeover effect and may delay, deter, or prevent a merger or acquisition of the Company by means of a tender offer, a proxy contest or other takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of Class A Common Stock held by stockholders.
Issuance of Undesignated Preferred Stock
Under the Certificate of Incorporation, the Board has the authority, without further action by the stockholders, to issue up to 100,000,000 shares of undesignated preferred stock, of which 10,000,000 shares were designated as Series A Preferred Stock, with rights and preferences, including voting rights, designated in the Certificate of Designation. When shares of Series A Preferred Stock are converted or otherwise reacquired by the Company, they will be promptly retired and not be reissued as shares of such series, but rather will become authorized but unissued shares of undesignated preferred stock. The existence of authorized but unissued shares of preferred stock would enable the Board to make it more difficult to attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise.
Classified Board
Our Certificate of Incorporation provides for a classified board consisting of three classes of directors, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. This provision may have the effect of delaying a change in control of the Board.
Election and Removal of Directors and Board Vacancies
Our Bylaws provide that directors are elected by a plurality vote. The Certificate of Incorporation provides that, subject to the rights of holders of preferred stock, unless otherwise provided by resolution of the Board approved by at least a majority of the total authorized directorships, only the Board may fill vacancies and newly created directorships on the Board. Directors may be removed only for cause by the affirmative vote of the holders of at least a majority of the voting power of the issued and outstanding capital stock entitled to vote in the election of directors. In addition, the number of directors constituting the Board may be set only by resolution adopted by a majority vote of the total authorized directorships. These provisions prevent stockholders from increasing the size of the Board and gaining control of the Board by filling the resulting directorships with their own nominees.
22
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our Bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors that specify certain requirements as to the timing, form and content of a stockholder’s notice. Business that may be conducted at an annual meeting of stockholders will be limited to those matters properly brought before the meeting. These provisions may make it more difficult for our stockholders to bring matters before our annual meeting of stockholders or to nominate directors at annual meetings of stockholders.
No Written Consent of Stockholders
Our Certificate of Incorporation provides that, subject to the rights of holders of preferred stock, all stockholder actions be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of the Bylaws or removal of directors by our stockholders without holding a meeting of stockholders.
No Stockholder Ability to Call Special Meetings
Our Certificate of Incorporation provides that, subject to the rights of holders of preferred stock, only the chairperson of the Board, the chief executive officer, the president or the Board, acting pursuant to a resolution adopted by a majority of the total authorized directorships on the Board, may be able to call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders.
Amendments to Certificate of Incorporation and Bylaws
Any amendment to our Certificate of Incorporation must be approved by the Board, acting pursuant to a resolution adopted by a majority of the total authorized directorships on the Board, as well as, if required by law or the Certificate of Incorporation, a majority of the outstanding shares entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of Section 3 of Article IV, Section 2 of Article V, Section 1 of Article VI, Section 2 of Article VI, Section 5 of Article VII, Section 1 of Article VIII, Section 2 of Article VIII, Section 3 of Article VIII or Article XI of the Certificate of Incorporation must be approved by not less than the affirmative vote of 66 2/3% of the total voting power of all the then-outstanding shares of stock. The affirmative vote of a majority of the Board or the holders of at least a majority of the voting power of all then-outstanding capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to amend the Bylaws, except that the amendment of Article VIII of the Bylaws must be approved by not less than the affirmative vote of 66.7% of the total voting power of all the then-outstanding shares of stock.
These provisions are designed to enhance the likelihood of continued stability in the composition of the Board and its policies, to discourage certain types of transactions that may involve an actual or threatened acquisition of our company and to reduce our vulnerability to an unsolicited acquisition proposal. We also designed these provisions to discourage certain tactics that may be used in proxy fights. However, these provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they may also reduce fluctuations in the market price of our shares that could result from actual or rumored takeover attempts.
Delaware General Corporation Law Section 203
As a Delaware corporation, we are also subject to the anti-takeover provisions of Section 203 of the DGCL, which generally prohibits a Delaware corporation from engaging in a “business combination” (as defined in the statute) with an “interested stockholder” (as defined in the statute) for a period of three years after the date of the transaction in which the person first becomes an interested stockholder, unless the business combination or the transaction by which the applicable stockholder became an interested stockholder is approved in advance by a majority of the independent directors or by the holders of at least two-thirds of the voting power of the outstanding disinterested shares. The application of Section 203 of the DGCL could also have the effect of delaying or preventing a change of control of us.
23
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, the Company’s stockholders have appraisal rights in connection with certain mergers, consolidations or conversions of the Company. Pursuant to the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger, consolidation or conversion will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of the Company’s stockholders may bring an action in the Company’s name to procure a judgment in the Company’s favor, also known as a derivative action, if certain conditions are met, provided that the stockholder bringing the action is a holder of the Company’s shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors and certain officers to corporations and their stockholders for monetary damages for breaches of directors’ and officers’ fiduciary duties, subject to certain exceptions. The Company’s governing documents include a provision that eliminates the personal liability of directors and officers for monetary damages for any breach of fiduciary duty as a director or officer, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of the Company and its stockholders, through stockholders’ derivative suits on the Company’s behalf, to recover monetary damages from a director or officer for breach of fiduciary duty as a director or officer in certain circumstances, including breaches resulting from grossly negligent behavior. However, exculpation does not apply to any director if the director has acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper benefit from his or her actions as a director and does not apply to officers if the officer has acted in bad faith, knowingly or intentionally violated the law or derived an improper benefit from his or her actions as a director or in the context of an action by or in the right of the Company.
The Certificate of Incorporation provides that the Company must indemnify its directors, and the Bylaws provide that the Company must indemnify and advance expenses to its directors and officers, to the fullest extent authorized by the DGCL. The Company is also expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for its directors, officers, employees and agents for some liabilities. The Company believes that these indemnification and advancement provisions and the authority to carry insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, advancement and indemnification provisions in the Governing Documents may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duty.
These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit the Company and its stockholders. In addition, your investment may be adversely affected to the extent the Company pays the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Transfer Agent and Registrar
The transfer agent and registrar for our Class A Common Stock and Public Warrants is Equiniti Trust Company, LLC (formerly known as American Stock Transfer & Trust Company, LLC).
Equiniti Trust Company, LLC
6201 15th Avenue
Brooklyn, New York 11219
Listing of Securities
Our Class A Common Stock and Public Warrants are listed on The Nasdaq Capital Market under the symbols “COCH” and “COCHW,” respectively.
24
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 (the “Registration Statement,” which term shall encompass all amendments, exhibits, annexes and schedules thereto and all documents incorporated by reference therein) pursuant to the Securities Act, and the rules and regulations promulgated thereunder, with respect to the securities offered hereby. This prospectus, which constitutes a part of the Registration Statement, does not contain all the information contained in the Registration Statement, parts of which are omitted in accordance with the rules and regulations of the SEC. For further information with respect to us and the securities offered hereby, reference is made to the Registration Statement.
We are required to file annual and quarterly reports, special reports, proxy statements, and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information regarding issuers, including us, that file electronically with the SEC. You may obtain documents that we file with the SEC at http://www.sec.gov. We also make these documents publicly available, free of charge, on our website at www.envoymedical.com as soon as reasonably practicable after filing such documents with the SEC. Please note, however, that information on, or accessible through, our website is not, and should not be deemed to be, a part of this prospectus and should not be relied upon in connection with making any investment decision with respect to the offered securities.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus, and information in documents that we file later with the SEC will automatically update and supersede information in this prospectus. We incorporate by reference into this prospectus the documents listed below and any future filings made by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act until we close this offering, including all filings made after the date of the initial registration statement and prior to the effectiveness of the registration statement. We hereby incorporate by reference the following documents:
| ● | Our Annual Report on Form 10-K for the year ended December 31, 2024, filed on March 31, 2025; |
| ● | Our Quarterly Reports on Form 10-Q for the quarter ended March 31,2025 filed on May 1, 2025; |
| ● | Our Current Reports on Form 8-K (excluding any reports or portions thereof that are deemed to be furnished and not filed) filed on May 19, 2025, June 3, 2025, June 25, 2025, July 2, 2025, and July 29, 2025; and |
| ● | The description of our Class A Common Stock contained in Exhibit 4.3 to our Annual Report on Form 10-K for the year ended December 31, 2023 filed April 1, 2024, including any amendment or report filed for the purpose of updating such description |
Any statement contained in a document, all or a portion of which is incorporated or deemed to be incorporated by reference herein, will be deemed to be modified or superseded to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified will not be deemed to constitute a part hereof, except as so modified, and any statement so superseded will not be deemed to constitute a part hereof.
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address:
Envoy Medical, Inc.
4875 White Bear Parkway
White Bear Lake, MN 55110
Phone: (877) 900-3277
Copies of these filings are also available, without charge, through the “Investors” section of our website (www.envoymedical.com) as soon as reasonably practicable after they are filed electronically with the SEC. Please note, however, that information on our website is not, and should not be deemed to be, a part of this prospectus.
25
The legality of the securities offered hereby have been passed upon for us by Morrison & Foerster LLP.
The audited financial statements incorporated by reference in this prospectus and elsewhere in the registration statement have been so incorporated by reference in reliance upon the report of Grant Thornton LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
26
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following table sets forth the various expenses in connection with the sale and distribution of the securities being registered. All amounts shown are estimates, except the SEC registration fee. The registrant has agreed to pay these costs and expenses.
| Securities and Exchange Commission registration fee | ||||
| FINRA corporate filing fees | * | |||
| Printing and engraving expenses | $ | 5,000 | ||
| Legal fees and expenses | $ | 15,000 | ||
| Accounting fees and expenses | $ | 10,000 | ||
| Transfer Agent and Registrar fees | * | |||
| Miscellaneous | * | |||
| Total | $ | * | ||
| * | Estimated expenses not presently known. The applicable prospectus supplement will set forth the estimated aggregate amount of expenses payable in respect of any offering of securities. |
Item 15. Indemnification of Directors and Officers.
Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, provides that a Delaware corporation, in its certificate of incorporation, may limit the personal liability of a director or officer to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director or officer, except for liability for any:
| ● | breach of the director’s or officer’s duty of loyalty to the corporation or its stockholders; |
| ● | act or omission not in good faith or which involve intentional misconduct or a knowing violation of law; |
| ● | a director for any unlawful payment of dividends or redemption of shares; |
| ● | transaction from which the director or officer derived an improper personal benefit; or |
| ● | an officer in any action by or in the right of the corporation. |
Section 145(a) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, so long as the person acted in good faith and in a manner he or she reasonably believed was in or not opposed to the corporation’s best interests, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action or suit by or in the right of the corporation to obtain a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action, so long as the person acted in good faith and in a manner the person reasonably believed was in or not opposed to the corporation’s best interests, except that no indemnification shall be permitted without judicial approval if a court has determined that the person is to be liable to the corporation with respect to such claim. Section 145(c) of the DGCL provides that, if a present or former director or officer has been successful in defense of any action referred to in Sections 145(a) and (b) of the DGCL, the corporation must indemnify such officer or director against the expenses (including attorneys’ fees) he or she actually and reasonably incurred in connection with such action.
II-1
Section 145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise against any liability asserted against and incurred by such person, in any such capacity, or arising out of his or her status as such, whether or not the corporation could indemnify the person against such liability under Section 145 of the DGCL.
Additionally, our Certificate of Incorporation eliminates our directors’ liability to the fullest extent permitted under the DGCL. The DGCL provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
| ● | for any transaction from which the director derives an improper personal benefit; |
| ● | for any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| ● | for any unlawful payment of dividends or redemption of shares; or |
| ● | for any breach of a director’s duty of loyalty to the corporation or its stockholders. |
If the DGCL is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of the Company’s directors will be eliminated or limited to the fullest extent permitted by the DGCL, as so amended.
In addition, we have entered into separate indemnification agreements with our directors and officers. These agreements, among other things, require us to indemnify our directors and officers for certain expenses, including attorneys’ fees, judgments, fines, and settlement amounts incurred by a director or officer in any action or proceeding arising out of their services as one of our directors or officers or any other company or enterprise to which the person provides services at our request.
We maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers.
II-2
Item 16. Exhibits.
The following is a list of exhibits filed as part of this registration statement.
| * | Filed herewith |
II-3
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
Provided, however, that the undertakings set forth in paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statements or is contained in a form of prospectus filed pursuant to Rule 424(b) that is a part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser:
(i) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
II-4
(B) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: (i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; (ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; (iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and (iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, and will be governed by the final adjudication of such issue.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement on Form S-3 to be signed on its behalf by the undersigned, thereunto duly authorized, in White Bear Lake, Minnesota, on July 29, 2025
| ENVOY MEDICAL, INC. | ||
| By: | /s/ Brent T. Lucas | |
Chief Executive Officer |
||
Each person whose signature appears below constitutes and appoints Brent T. Lucas his or her true and lawful attorney-in-fact and agent with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments and supplements (including pre-effective and post-effective amendments) to this Registration Statement on Form S-3 of Envoy Medical, Inc., and any related Rule 462(b) registration statement or amendment thereto and other instruments necessary or appropriate in connection therewith, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents (and each of them acting singly) full power and authority to do all such things in his or her name and on his or her behalf in their capacity as an officer and/or director to enable Envoy Medical, Inc. to comply with the provisions of the Securities Act of 1933, as amended, and all requirements of the Securities and Exchange Commission, hereby ratifying and confirming all that said attorneys-in-fact, or any of them or their agents, substitutes or resubstitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated:
| Signature | Title | Date | |||
| /s/ Brent T. Lucas | Chief Executive Officer and Director (Principal Executive Officer) | July 29, 2025 | |||
| Brent T. Lucas | |||||
| /s/ Robert Potashnick | Interim Chief Financial Officer (Interim Principal | July 29, 2025 | |||
| Robert Potashnick | Financial and Accounting Officer) | ||||
| * | Director | July 29, 2025 | |||
| Chuck R. Brynelsen | |||||
| /s/ Michael Crowe | Director | July 29, 2025 | |||
| Michael Crowe | |||||
| * | Director | July 29, 2025 | |||
| Glen A. Taylor | |||||
| * | Director | July 29, 2025 | |||
| Mona Patel | |||||
| * | Director | July 29, 2025 | |||
| Janis Smith-Gomez | |||||
| * | Director | July 29, 2025 | |||
| Susan J. Kantor | |||||
| *By: | /s/ Brent Lucas | Attorney-in-Fact | July 29, 2025 | ||
| Brent Lucas | |||||
II-6
